Published on LinkedIn: https://www.linkedin.com/pulse/when-ok-cheat-win-business-oliver-canty



In a letter published today in the Journal of Hospital Infection, Professor Peter Wilson and colleagues report on their retesting of the Deprox (Hygiene Solutions Ltd) HPV decontamination system. The retesting was in response to concerns widely raised that the earlier tests of the system, which purported to give a log 6 efficacy, were the result of the manufacturer misrepresenting the concentration and/or constituents of the Deproxin solution.
In brief, the retest reveals the following:
It is very much to the credit of Professor Wilson and colleagues that they have thoroughly retested the system in response to widespread concerns. It is sad that commercial interests would abuse the trust and confidence of highly qualified academics in this way by misrepresenting the basic test parameters.
For those without access to the Journal of Hospital Infection, I have reproduced the letter below, and a PDF is downloadable here.
Sir,
In response to the letter from Dr Singh commenting on our paper.[1], [2]
The objective of our study was to evaluate the reductions in environmental contamination during in-use operation of two commercially-available hydrogen peroxide whole-room disinfection systems.2 Both manufacturers agreed test parameters prior to the trial to ensure methodology followed manufacturer instructions. Our findings suggested similar efficacy of the two systems against both surface contamination and biological indicators of common pathogens. Inocula used on the indicators far exceeded the likely levels seen in the environment.
Additional studies were performed as part of the original work using the same methodology with four strains each of MRSA, Klebsiella pneumoniae, Clostridium difficile spores and Acinetobacter baumannii. Three HPV decontamination cycles were evaluated for each system. Of 305/320 samples, >4-log10 reduction was achieved.
Aerial concentrations of hydrogen peroxide and relative humidity were monitored continuously during a further 6 cycles of both systems using a sensor (C-16 Portasens II Gas Detector; Analytical Technology Inc., Collegeville, PA, USA). In addition, horizontal surfaces in the near-patient vicinity were swabbed and analysed to detect fallout of silver and nitrate at the end of HPV decontamination cycles (n=3). Surfaces were swabbed and analysed for silver by titration (Silver Test Kit, DTK Water, Wellingborough, UK) and nitrate using Quantofix semi-quantitative test strips (Macherey-Nagel, Düren, Germany).
For the Deprox (Hygiene Solutions, King’s Lynn, UK) system, peak aerial values of 29-46 ppm hydrogen peroxide were achieved with similar bacteriological efficacy as other cycles. The mean level at the end of the cycles was 3.3ppm for 41.8% (30.8-58.1%) mean relative humidity at start of cycles. Silver and nitrate were detected on surfaces at 1.5-2.5mg/m2 following cycles with the Deprox system.
For the Bioquell Q10 system with the R10 aeration unit (Bioquell, Andover, UK), the peak aerial levels of hydrogen peroxide were 450-640ppm. The mean level at the end of the cycles was 0.0ppm with starting mean relative humidity 42.5% (34.5-49.7%). No silver or nitrate was detected on surfaces following cycles with the Bioquell Q10 system.
The aqueous concentration of hydrogen peroxide in a Hygiene Solutions cartridge (Deprox) tested on one occasion at the point of insertion into the machine was 5%. Nitrate was detected in the aqueous solution at 10-25mg/L. The aqueous hydrogen peroxide concentration in the Bioquell Q10 cartridge (Bioquell HPV-AQ) was 35% and no silver or nitrate was detected.
Dr Singh suggests C. difficile spores (but not the other organisms) persisted underneath the bed and on the window frame after decontamination using the Deprox system. The persistence of spores may have been minimised during the Bioquell Q10 cycles by the inclusion of an oscillating fan to facilitate aerial distribution and aid breakdown of hydrogen peroxide vapour.
As Dr Singh suggests, settling of active silver onto biological indicator coupons during a cycle of aerial HPV decontamination may have contributed to the bactericidal/sporicidal activity of the Deprox system. However further studies would be required to elucidate its role.

Incredibly, so-called “Hygiene Solutions Ltd” whose Deprox and Ultra-V machines are wheeled in to intensive care units and operating theatres on a daily basis, operates from the back of a dirty farming and livestock supplies warehouse, belonging to D&H Animal Husbandry. Here the Deprox units of their “On-call decontamination service” are stored between call-outs alongside pails of cattle feed and assorted agricultural and veterinary detritus.
Here is another view of the real Hygiene Solutions. I have added some notes for the non-technical reader.
The covert camera video below gives a good idea of the grubby reality behind Hygiene Solutions polished sales presentations:
https://www.dropbox.com/s/jtg3hztrh2cki32/warehouse%20tour.avi?dl=0
These less than ideal surroundings are fertilised by a regular stream of D&H customers, arriving “fresh” from their chicken sheds, stables, pig barns and cow pastures, who trek malodorously through “Hygiene Solutions Ltd” to the trade counter at the back of the barn.

Judge Adele Williams
Judgement was pronounced on a £26,000 NHS fraud last week. The perpetrators only escaped jail as they had pleaded guilty at the earliest opportunity and had repaid the money in full.
But Judge Adele Williams described it as a
She said further: –
What would the Judge have to say to the perpetrators of an £8,000,000 fraud against the NHS? Even if the directors of Hygiene Solutions were to refund the NHS for the tens of thousands of fraudulent Deprox processes they have done over the years, (see https://deproxfraud.info/2017/03/13/leaked-emails-prove-test-cheating-bodily-harm-and-massive-fraud/ ) would they escape jail?

Hygiene Solutions claim a single Deprox unit has the capacity to decontaminate rooms with a volume of 380m3, e.g. a 12 bed ward bay. A typical hospital side ward (single room with ensuite) has a volume of 60m3.
However, the chamber used by Hygiene Solutions Ltd to test the Deprox is 1.5m x 1.5m x 2.8m. Total volume 6.3m3 , just 10% of the volume of a hospital single bedroom, and 1.7% of the maximum volume that Deprox is guaranteed to disinfect. It is barely larger than a telephone box.
Hygiene Solutions internal testing, published here for the first time reveals that the Deprox, in spite of being boosted with 50% more concentrated hydrogen peroxide solution than the standard “Deproxin” was incapable of a log 6 decontamination of even this tiny test chamber.
The Deprox was thoroughly tested over a period of months by David Sempere Aracil, a well qualified chemist. David placed Log 6 biological indicator discs (Apex Biological Indicator #HMV-091) in 8 different locations around the inside walls of the test chamber. The Deprox unit (“Trusted by leading hospitals around the world”) was sealed in the chamber, and the process was run. The log6 BIs were incubated – they were all alive.
David tried substituting Sanosil SO15 which at 7.5% H2O2 is 50% more concentrated than Deproxin. Now some of the BIs would be sterilised, sometimes. Over several weeks in late 2014, David did a series of 12 tests in the test chamber, all with 7.5% H2O2 rather than the 5% Deproxin. He tried turning the ΔRH up and down, but to no avail. In 5 of these tests, all 8 BIs remained viable. None of the tests sterilised more than 6 out of 8.
In summary then:
Deprox, running on a 5% H2O2 solution, is claimed to give a log6 decontamination of an entire 380m3 ward, including inside small crevices and complex equipment. In Hygiene Solutions’ own tests, the Deprox running on a 7.5% solution, and thus generating a 50% higher aerial H2O2 concentration than the standard process, completely failed to give a log6 decontamination of a 6.3m3 box in multiple tests.
Hygiene Solutions continued to promote and sell the Deprox with exactly the same claims, but in 2015, they turned the whole Deprox fleet down from ΔRH20 to ΔRH5. See https://deproxfraud.info/2017/03/13/leaked-emails-prove-test-cheating-bodily-harm-and-massive-fraud/
Fortunately, (or unfortunately for Hygiene Solutions) David’s notes of these tests survived.
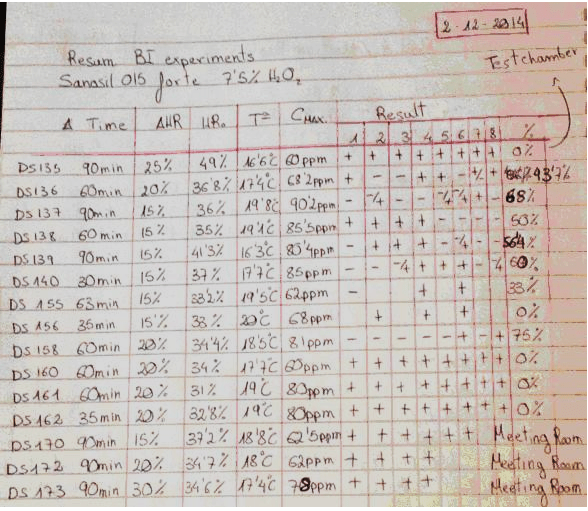
This table is a summary of 15 tests done by David Sempere Aracil, assisted by Tautvydas Karitonas, over a period of months. Both are university graduates with extensive research experience, and David has a PhD in Chemistry. The tests were done with a standard production model Deprox machine, the purpose of the tests was to determine if the extremely low efficacy of the Deprox process could be rectified by increasing the concentration of the hydrogen peroxide solution from the standard 5% to 7.5%.
The results were recorded in 3 A4 hardcover notebooks. Each of the 15 tests was recorded in more detail on preceding pages of the notebook. In addition to the efficacy tests, the notebooks contain extensive details of tests on prototype catalyst systems, and constitute proof that HS was well aware of both the low efficacy and residual gas issues with Deprox.
Heading: “Sanosil 015 forte” refers to Sanosil S015, which is a disinfectant intended for water systems. It is 7.5% Hydrogen peroxide solution. Note that this is more concentrated than the 5% Deproxin solution that is used in production Deprox machines.
Col.1. The test number. These are not sequential, as some tests did not use Biological indicators (BIs) and were not recorded in this resume.
Col. 2 Duration of test measured from when the machine starts vapourising. (It takes several minutes for the machine to fill the piezo tank at the beginning of each test)
Col. 3 Delta HR setting of machine. This is adjusted by using unmarked pressure sensitive switches below the LCD display. – see How to test your Deprox.
Col. 4 HRO This is the original relative humidity in the test chamber before the machine starts.
Col. 5 TO Temperature in the chamber before the machine starts
Col. 6 CMAX Hydrogen peroxide concentration in PPM, maximum level reached during process.
Results columns. The first 12 tests were done in the test chamber (wardrobe). Each number represents a specific marked location on the test chamber wall where an exposed stainless steel Bacillus subtilis log6 biological indicator was placed. The chamber is a crude wood and plasterboard structure in an essentially unheated warehouse. It is approximately 5’ x 5’ x 9’ and the indicators were placed at various heights on the interior walls of the chamber. The last 3 tests were done in the company board room which is approximately 12’ x 25’.
A” +” indicates that the BI still contained viable bacteria, a “–“ indicates that all bacteria on this indicator were killed.
Final column. This is the percentage of BIs that were killed.

Or should the question be, is ANYTHING inside your Spectrome? Following recent posts urging Ultra-V users to do some simple tests on their equipment, I have received several very remarkable reports. It appears that the Ultra-V will run a full cycle and “validate” that a hospital room has been successfully decontaminated even with two of the Spectromes placed outside of the door!
Either the special brand of UV light that the Ultra-V produces can pass through 50mm of wood, (in which case it is a grave danger to anyone in the building) or else the Spectromes are not really monitoring the UV output at all. Here is what Hygiene Solutions Ltd. claim for their “Patented Spectrome Technology”.
Sorry – the quote above is verbatim. We know what they meant to say…
How exactly does the Spectrome “evaluate” the “extent of bacteria”? This is truly remarkable technology! And all this without even being in the room!
As the crocodile says, “Becuss it printed right dere in words, dat make it true.”
What is Spectrome indeed? I have a suspicion it might just be an empty box…

Move over Wikileaks – now we have Ricki-leaks…
A large batch of very interesting internal Hygiene Solutions emails have fortuitously come into my possession. Too much material for one post so this will just be the first of three…
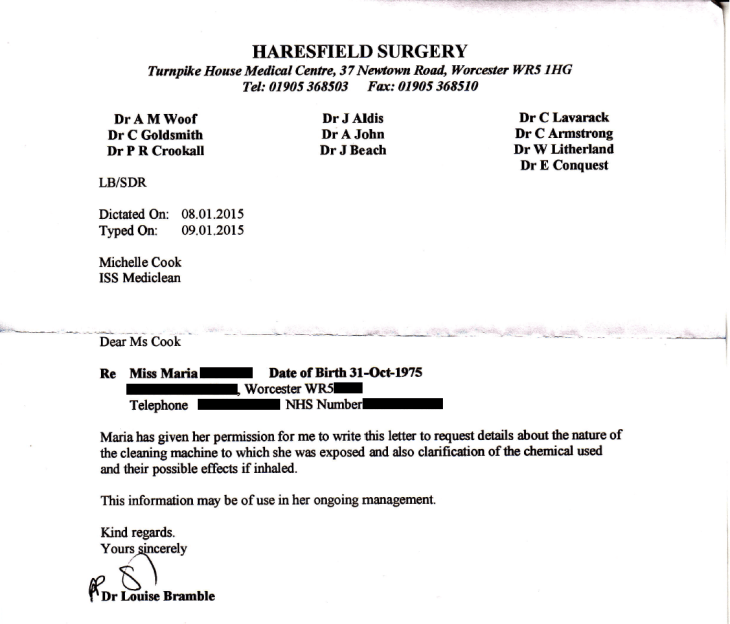
In January 2015, Mark Fentiman sent an urgent message that all Deprox units were to be turned down to RH5. Now it emerges that this order was in response to the incident above, in which a healthcare worker at the Royal Worcester Hospital inhaled Deproxin fumes and suffered serious respiratory problems. (Note that ISS Healthcare operate the Deprox machines as a subcontractor in some NHS hospitals.)

The Log6 claim so loudly and insistently trumpeted by Hygiene Solutions is based on a machine set to RH40. The RH setting gives the amount of H2O2 per cubic metre of air, so resetting the machine to RH5 only gives 12% of the required concentration. In reality, the machine is just a placebo – no useful or meaningful level of disinfection is possible at this level.
The shocking fact is that Hygiene Solutions continued to provide their Deprox systems and “decontamination” service at this dangerous and utterly ineffective level, did not inform their 60 or so NHS Hospital customers of the change, and continued to charge full price, a tidy sum of £2,500,000 per year.
Given that approximately 5000 NHS patients die each year from the very infections that this system is supposed to prevent, there can be no doubt that this action led to completely avoidable infections and death, as well as robbing the NHS of millions of pounds in fees for thousands of imaginary decontamination services that did not actually take place.
To follow:

The Deprox settings can be simply tested as follows:
Changing the RH setting changes the efficacy of the process. The efficacy is proportional to the H2O2 concentration, which is in turn proportional to the RH setting. The test data that Hygiene Solutions include in their tender submissions (see TNO Report ) are for machines running at delta RH40. The log 6 efficacy claims for Deprox are based only on this data.
However, at delta RH40, the level of H2O2 and other chemicals in the air at the end of the process is perhaps 20 or 30 times the legal limit – even after the 45 minute “deactivation cycle”. Also, except in the very driest climates, heavy condensation is formed. Consequently all Deprox units have been turned down to RH settings of between 5% and 15%. (For example, the 2 machines at Westmead Hospital in Sydney are set at 10%. The units at the Luton and Dunstable Hospital are set at 5% etc…) The efficacy is consequently greatly reduced, to between log 0.2 and log 2.
It should be pointed out that the Deprox machine has NO MEANS WHATEVER of monitoring the deactivation process. The green light is simply on a 45 minute timer, and will illuminate at this time regardless of the level of gas in the room.
Hydrogen peroxide gas detectors are expensive to buy, but can be rented at very reasonable rates from Drager UK. The best detector for this application is the Drager X-am 5100. The contact number for gas detection enquiries is 01670 352891.
Note: Do NOT rely on on gas detectors supplied from Hygiene Solutions – these are calibrated to show only a fraction of the real gas level! This can very easily be demonstrated by using a HS supplied unit and a rented Drager unit side by side.
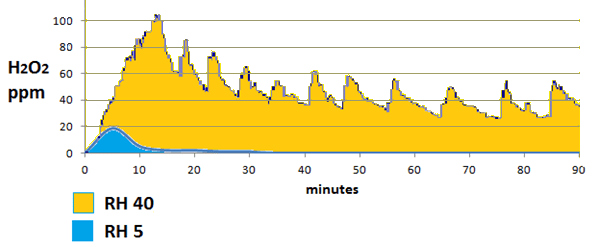
We can see from the graph above the huge reduction in both H2O2 concentration and duration between Deprox settings of RH40% and RH5%. For technical reasons explained in “Deprox and the Emperor’s New Clothes” at RH5% the Deprox only emits a single pulse of vapour, rather than emitting at 5 to 10 minute intervals as it is designed to do. The efficacy is approximately proportional to the area under the graph, so it is obvious that the reduction in efficacy is not 8 fold as we might calculate, but more like 100 fold.
Hygiene Solutions keep a couple of “demo” machines at their depots both in the UK and Australia that are set to a delta RH of 30%. These are used to “demonstrate” to new or skeptical customers that the process is efficacious. As the H2O2 has little odour, the residual air quality issue is not apparent unless an H2O2 meter is used.
For full details on the delta RH setting deception, and its consequences, see “Deprox and the Emperor’s New Clothes”
Specialist Hygiene Solutions Australia Pty Ltd, the sponsors for the Deprox and Deproxin products in Australia has sunk. The ABN has been cancelled, and the company no longer exists. There are thought to be about 20 units in Australia, as follows:
As Deprox has no Australian sponsor, they can no longer be legally used.
Sources inside the UK manufacturer, Specialist Hygiene Solutions Ltd, say that the Australian and New Zealand machines are only serviced on an “ad hoc” basis as there are limited servicing facilities in Australasia. Certainly the service records show that some of these machines have not been serviced for over a year, (see Deprox Fleet Report last 3 pages). This will cause poor fogging performance and increasing lead oxide contamination as the piezo discs erode, see https://deproxfraud.info/toxic-cocktail-lead-and-silver-nitrate/