Gordon’s Story
Gordon Cunningham started working for Hygiene Solutions early in 2013, building and servicing the Deprox machines, as well as operating the system in hospitals across the UK. He noticed that at the end of a decontamination process, the treated rooms often still had a visible white mist in the air, although the Deprox remote control light indicated that the room was safe to enter. He raised his concerns with company directors Rick and Mark Fentiman, but was told that there was nothing to worry about and that the process was entirely safe. Nonetheless, he requested a respirator and a hydrogen peroxide (H2O2) gas monitor (Draeger) to protect himself when using the Deprox, but his request was ignored.
Gordon, a non-smoker who keeps fit by running and triathlons, began to experience a tender sensation in his throat after being exposed to the Deprox residual vapour. These symptoms progressed to an asthma like feeling of a constricted airway and a hoarse cough.
The business owners, Rick and Mark Fentiman insisted that re-entering a treated room was safe, as long as the H2O2 level was below 5.5ppm. (In fact, the safe exposure level is 1ppm)
Gordon then spoke to the chemist who was working on the Deprox project, David Sempere Aracil. David told him that he should not be entering rooms at 5.5ppm.
In June 2014, Gordon was asked to spend 3 days operating the Deprox system at the Luton and Dunstable Hospital. Six months had passed since he requested a respirator but his request had not been responded to. When re-entering the rooms, he would try to cover his mouth and get the windows open as quickly as possible, to minimize his exposure. Gordon noticed one of the technicians had a new Draeger H2O2 meter, and he asked how it was operated. The technician explained how to use the meter, and how to set it so that it would sound an alarm buzzer until the gas concentration had dropped to a safe level. Gordon had done some research, so he knew the safe level was 1ppm. Gordon took the Draeger to Luton with him.
On Monday June 2nd 2014 Gordon had two rooms for Deprox treatment at the Luton and Dunstable Hospital. The first room was the equipment library. He set up a Deprox unit in this room, and 1 ½ hours later the green light on the Deprox remote control lit, indicating that the room was safe to enter. He un-taped the door, and followed the instructions from the technician, used the Draeger to check the gas concentration – it was 7.8ppm. Gordon left the Draeger in the room and taped the door closed again. Further to the technician’s instructions, there will be a continuous alarm tone until the safe level of 1 ppm was reached, the room will then be safe to enter.
Later in the day, some hospital staff wanted access to the library to get some equipment. Gordon explained that it was not safe to enter until the Draeger had indicated a safe level.
Four hours after the process had completed, the room was still inaccessible, as the Draeger was still giving an alarm tone. Gordon was summoned to the office of Camilla, head of Domestic Services. Camilla demanded to know what the problem was in the library, pointing out that in the Hygiene Solutions Deprox literature the “deactivation” cycle is only 90 minutes, and that they never normally had to wait longer than that. Gordon explained about the Draeger, and that the equipment library was not yet a safe environment.
A little later Gordon was summoned to the office again, and told that “Your boss, Mark Fentiman is on the phone, and says you are to take the Draeger out of the library immediately.” Mark Fentiman then phoned Gordon directly and ordered him to remove the Draeger and open up the library to the hospital staff.
Gordon un-taped the door and entered the room. The H2O2 level according to the Draeger was at 4.3ppm, well above the safety limit. Gordon removed the Draeger and opened up the room, telling the hospital staff to wait as long as possible to let the gas disperse.
Hygiene Solutions told the hospital that Gordon was using equipment that was not calibrated, and that he had not been trained on, and that the gas levels in the room had been completely safe. In fact, the meter was freshly calibrated, and Gordon has copies of the all calibration certificates to prove it.
Other staff members, including Tim Murrell, the Deprox patent holder, witnessed Mark Fentiman’s fury at the news. Tim said he had “never seen someone so angry” as when Mark found out that Gordon had taken a Draeger to Luton.
Gordon lost his job that week. The other staff at Hygiene Solutions were told it was because he used an uncalibrated meter without permission.
Gordon still suffers from respiratory problems as a result of his exposure to the Deprox fog. It may well be that inhaling the combination of hydrogen peroxide and silver nitrate has caused irreversible damage to his trachea and lungs.

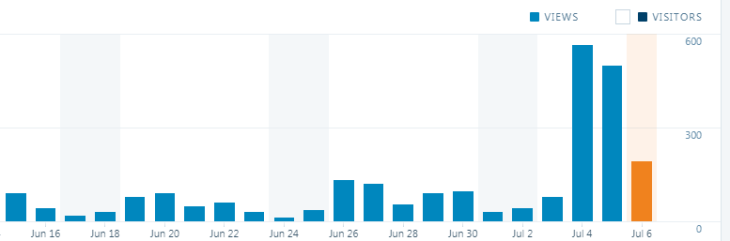
Update: http://www.deproxfraud.info achieved 40,000 views as of this afternoon, and now ranks higher on Google than Hygiene Solutions’ own website!