A letter sent today to all 308 delegates to the 2017 Australasian Conference on Infection Prevention and Control.
Dear Delegate,
I wish to draw your attention to the presence of Hygiene Solutions Ltd at ACIPC17. This UK company presents a serious threat to healthcare providers in Australia and New Zealand . Extensive evidence from both published papers and from disclosures by former employees of the business has revealed numerous fraudulent practices and reckless and irresponsible actions by this company. One well documented example is a very substantial and clearly correlated step increase in C. difficile infections in the University College London Hospitals on the implementation of the Deprox system, followed by an equally clear decrease when this system was abandoned 2 and a half years later. Approximately 70 additional cases over baseline occurred over the 29 months of Deprox operation. See: https://www.linkedin.com/pulse/freedom-information-request-reveals-exact-c-richard-marsh/
In brief, the history of these issues is as follows:
In response to insoluble technical problems with residual H2O2 vapour, the system manufacturer, Specialist Hygiene Solutions Ltd, secretly and drastically cut the disinfectant vapour concentration settings on the entire fleet of almost 200 systems, leaving the process almost entirely ineffective against the deadly HCAI pathogens it was claimed to eliminate. See:
https://deproxfraud.info/2017/03/13/leaked-emails-prove-test-cheating-bodily-harm-and-massive-fraud/
Thus not only were tens of thousands of patients needlessly put at risk of debilitating and possibly fatal infections, but the health services of the UK, Australia and New Zealand were defrauded of over £20,000,000.
Further to this, the Deproxin solution contains silver nitrate, which is forbidden in the EU for fogging applications on account of its toxicity to lung tissue and mucous membranes. Several Deprox operators have suffered chronic respiratory illness as a result of being exposed to the residual fog after a room has been treated. These persons are actively seeking legal compensation for these injuries.
See: https://deproxfraud.info/2017/11/07/2-more-deprox-operators-hit-with-throat-and-lung-damage/
The UK market is now largely aware of the dangerous and fraudulent nature of Hygiene Solutions Ltd, which has crippled their sales prospects in that area – hence their presence at ACIPC17 in an attempt to open up the Australasian market.
My simple request is that any healthcare institution contemplating doing business with Hygiene Solutions Ltd should thoroughly research the health and safety, and the germicidal efficacy of the products offered. Independent confirmation should be sought, as the directors of the company have no hesitation about fabricating evidence.
No less than 39 examples of these fabrications can be found here:
https://deproxfraud.info/deprox-fraud/
The Health and Safety Executive is leading an investigation in to Hygiene Solutions Ltd in the UK. The officer coordinating the operation is Mr Martin Ball;
Martin Ball | HM Inspector of Health and Safety
CRD Compliance Team
Health & Safety Executive Chemicals Regulation Division
5S.1 Redgrave Court, Merton Road, Bootle, Merseyside, L20 7HS.
Tel: 0151 951 3512
martin.ball@hse.gov.uk
Summary of issues
- The process is only capable of a small fraction of the claimed disinfection efficacy.
- Rooms and equipment cleaned with Deprox remain dangerously contaminated with pathogens, and are a serious and potentially lethal threat to patients.
- Residual chemicals of H2O2, AgNO3 and PbO are way in excess of legal limits and several operators have suffered permanent respiratory damage.
- The fraud is accomplished by a complex and sophisticated web of misinformation, outright lies, rigged tests, and claiming test results from competitor’s machines as their own.
- Healthcare providers in the UK, AU and NZ are being defrauded of tens of millions of pounds.
Sincerely,
Richard Marsh
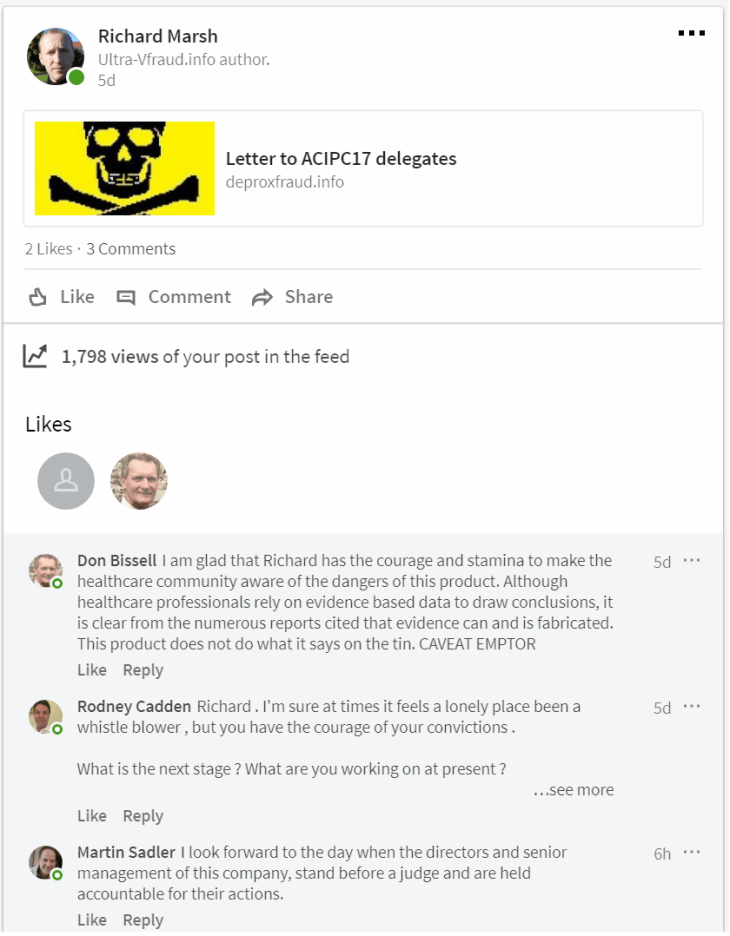
Response to my letter to #ACIPC17 Delegates, from an administrator within the North Sydney Local Health District

Hygiene Solutions posts fake email addresses at ACIPC17
Hygiene Solutions has two new e-publications for ACIPC17, these are in the Sponsor e-satchel section on the ACIPC17 app. ( https://guidebook.com/guide/102693/poi/8908501/ )
All but one of the website addresses given on these documents is fake:
Only the UK website actually exists: